By James G. Martin, author of
Revelation through Science
Is DNA and its relationship to RNA and other organic molecules a miracle? This article explores a compelling example from organic chemistry based on the three-dimensional structures of certain sugars, proteins, and DNA and RNA; and how each of these depends on all the others. Could any of these exist without the others? Could this happen by unguided chance, even in 4.5 billion years?
Four hundred years ago, the theological academies and hierarchies were rocked by Galileo’s findings that Venus orbited the Sun, the Moon’s visage was pock-marked, and Jupiter had four moons. Peaceful accommodation slowly settled in, only to be wracked again 250 years later by Darwin’s evolutionary explanation of “The Origin of Species.” When atheists seized upon Darwin’s theory as a rejection of God, fundamentalist Christians countered by rejecting evolution. Following the famous 1925 Scopes “Monkey Trial” in Dayton, Tennessee, US Courts have steadily blocked any requirement to teach religious tenets against science in public schools.
These and related historic episodes are recounted in Revelation through Science, along with various dodges, such as the curious planetary concept of Tycho Brahe that the Sun and Moon orbit around the stationary Earth, while all other planets revolve around the Sun. Many theologians and scientists attempted to resolve matters with a duality principle: that science and religion are non-overlapping domains with separate but equal access to truth.
Scientists Point to Creation by Powers that Transcend Nature
In recent decades, however, a group of theistic astronomers, physicists, paleontologists and physicians have raised a fascinating theme that many fundamental concepts of science point to creation by powers that transcend and predate nature. Among these are the “Big Bang” origin of the universe, with highly precise values for the physical constants for (a) gravity, (b) the strong nuclear force, (c) the weak nuclear force, and (d) the electromagnetic force. Together, these comprise a coordinated set of fine-tuned, “anthropic coincidences” leading to the conclusion that life is either highly conditional upon a foreordained purpose ─ or it is extremely lucky. This modern reconciliation between science and religion flourished with the publication of The Language of God, by N.I.H. Director Francis Collins, MD, former head of the “Human Genome Project.” Its title is an obvious and intentional metaphor for the built-in growth and control of our bodies and lives by the enormous yet explicit code within our DNA. This supports both a mechanistic proof of evolution and a belief that evolution had to be purposeful.
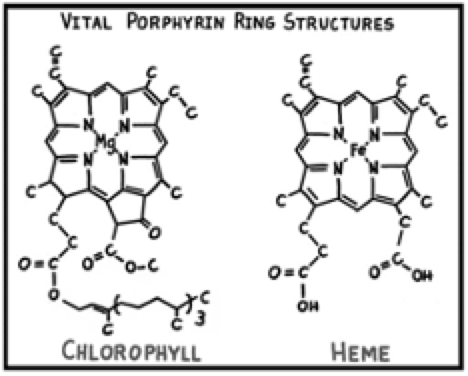 Believing that similar “evidence for belief” abounds in other fields of science, I have a series of chapters about astronomy, physics, geology, biology, chemistry and biochemistry, each with its own anthropic coincidences. The earlier sections are to some extent reflections of similar accounts from previous authors, but Revelation Through Science is the first with provocative examples from my field of organic chemistry, and they are numerous and compelling. There are a number of extremely complex naturally occurring compounds like hemoglobin that defy laboratory synthesis, or chlorophyll, which has been synthesized in five years by Nobel laureate Robert Woodward and his large team of Harvard chemists. Of course, ordinary leaves can do this any sunny day, not only routinely, but far more proficiently, albeit by incredibly sophisticated processes.
Believing that similar “evidence for belief” abounds in other fields of science, I have a series of chapters about astronomy, physics, geology, biology, chemistry and biochemistry, each with its own anthropic coincidences. The earlier sections are to some extent reflections of similar accounts from previous authors, but Revelation Through Science is the first with provocative examples from my field of organic chemistry, and they are numerous and compelling. There are a number of extremely complex naturally occurring compounds like hemoglobin that defy laboratory synthesis, or chlorophyll, which has been synthesized in five years by Nobel laureate Robert Woodward and his large team of Harvard chemists. Of course, ordinary leaves can do this any sunny day, not only routinely, but far more proficiently, albeit by incredibly sophisticated processes.
Could DNA, RNA and other Complex Molecules Evolve only by Chance?
My most compelling example from organic chemistry is based on the three-dimensional structures of certain sugars, proteins, and DNA and RNA; and how each of these depends on all the others. Could any of these exist without the others? Could this happen by unguided chance, even in 4.5 billion years? What you think about this enigma and choose to believe is up to you. But consider this.
All natural proteins consist of long polymeric chains of the same set of 20 amino acids. Much has been made of the fact that many amino acids have been found in meteorites, and can even be formed by lightning discharges in an atmosphere that probably existed when Earth was sterile ─ before the first living organisms 3.5 billion years ago. It was typically implied that these amino acids could somehow self-assemble into complex proteins. These would coalesce into clusters, which would evolve into living cells. Knowing today how proteins are biosynthesized in cells, we can see that it is far more complicated than that. First of all, let me introduce you to a phenomenal relationship, between two small but essential sugars, the proteins that catalyze their biosynthesis, and the peculiar structures of DNA and RNA. We can then unfold and examine an extraordinary structural co-dependence between them, just as unfamiliar to most readers as it is miraculous in its improbability ─ leaving us to wonder how nature ever got it precisely correct. Think of it as the most profound three-way “chicken and egg” conundrum: which came first?
 The simple sugar known as ribose is a vital part of the helical backbone of ribonucleic acid (RNA). Its cousin deoxy-ribose is a vital part of the backbone of the double helix of deoxy-ribonucleic acid (DNA). Both of these simple sugars are biosynthesized in cells by certain proteins. To complete this puzzle, all proteins are biosynthesized in cells. For each of some 23,000 distinct human proteins, the defining sequence of amino acids is predetermined by the genetic code carried by its unique RNA. The RNA that is specific for one protein gets its code directly from one of 23,000 genes in the vast catalog of codes built into DNA.
The simple sugar known as ribose is a vital part of the helical backbone of ribonucleic acid (RNA). Its cousin deoxy-ribose is a vital part of the backbone of the double helix of deoxy-ribonucleic acid (DNA). Both of these simple sugars are biosynthesized in cells by certain proteins. To complete this puzzle, all proteins are biosynthesized in cells. For each of some 23,000 distinct human proteins, the defining sequence of amino acids is predetermined by the genetic code carried by its unique RNA. The RNA that is specific for one protein gets its code directly from one of 23,000 genes in the vast catalog of codes built into DNA.
Among the structural components of every molecule of RNA and DNA is the particular sugar, ribose or deoxy-ribose respectively. Human DNA has thousands of genes, 23,000 of which carry the codes for each of 23,000 vital proteins, Each gene code is transcribed to a particular RNA molecule. It translates this code into the sequential selection of 20 different amino acids (by the hundreds) for its particular protein. Among these proteins are some that facilitate replication of DNA code prior to cell division, so that each new cell has the same code. Other proteins guide the biosynthesis of ribose and deoxy-ribose. Without these two sugars, there could be no DNA or RNA. Without RNA having the code from protein-coding genes of DNA, there could be no functioning proteins. Without carbohydrate-synthesizing proteins, there could be no sugars. They are mutually and existentially interrelated.
Each Life-Giving Compound is Wholly Interdependent with Each and All the Others
You see, each of these life-giving compounds is wholly interdependent with each and all of the others. Which came first? It seems to me that all had to be present in the very first living cell, or it could never reproduce its replica living cell.
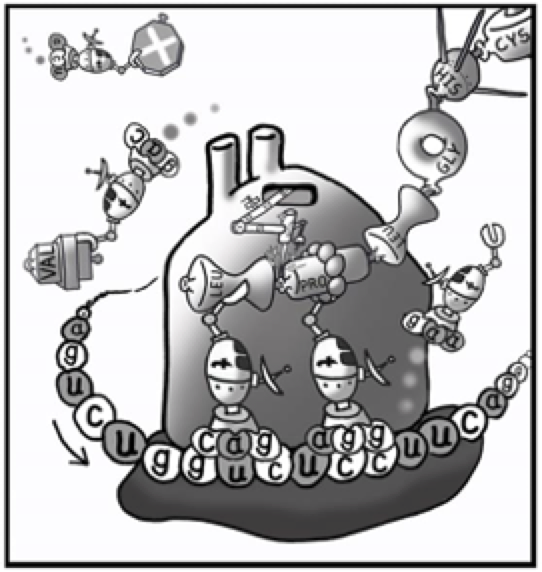 This next cartoon tries to simplify the mental image of this, without getting lost in the elaborate actual molecular structures of the various compounds. The protein factory, called a ribosome, is represented by a blob of a giant protein with suggestions of smoke stacks to help you accept that however complex it is, it is basically a kind of factory. Little machines called transfer-RNA are running around inside the cell with a short code that allows each t-RNA to select one specific amino acid. Finally, there is a chain of codes representing messenger-RNA, riding across the bottom of the ribosome, with the entire chemical code for this one protein. Each three-part sequence of its code can only match one specific t-RNA, thus keeping absolute control of the intended sequence of amino acids being connected by the ribosome as it constructs the new protein.
This next cartoon tries to simplify the mental image of this, without getting lost in the elaborate actual molecular structures of the various compounds. The protein factory, called a ribosome, is represented by a blob of a giant protein with suggestions of smoke stacks to help you accept that however complex it is, it is basically a kind of factory. Little machines called transfer-RNA are running around inside the cell with a short code that allows each t-RNA to select one specific amino acid. Finally, there is a chain of codes representing messenger-RNA, riding across the bottom of the ribosome, with the entire chemical code for this one protein. Each three-part sequence of its code can only match one specific t-RNA, thus keeping absolute control of the intended sequence of amino acids being connected by the ribosome as it constructs the new protein.
You will notice that I slipped in the adjective, “intended,” because I believe this process is far beyond anything that could just happen by trial-and-error collisions of lifeless molecules. In all the annals of evolutionary biology, none of this works if there is no DNA with its thousands of specifically coded genes. Nothing like it works if there is no biosynthesis of not only these two sugars, but also the thousands of diverse, biologically active proteins. You are free to reach your own conclusion and belief as to whether life with this kind of complexity could ever occur by unguided chance, or was intended.
Nature has a Mysterious Preference for Left-Handed Molecules
Would you allow me to introduce an even more amazing “miracle,” if I may call it that? Again, you will be free to see if it fits your personal belief system when I finish. You may want to rest your mind a bit before proceeding. Take a moment for a few deep breaths. Relax your jaw. Massage your forehead. When you are ready, consider this.
Most biologically active organic compounds are asymmetrical in three-dimensional space. Because of their asymmetry, they could possibly exist as two hypothetical forms that are ”nonsuperimposable” mirror images of each other. Like your hands (and feet) they may be what chemists have classified as right-handed molecules, or they may be left-handed. Look at the accompanying illustration below. (Like the others, it was created by our son, Jim Martin Jr. He even added the clever metaphor of a gloved hand just to help you keep track.) Each amino acid has one key carbon atom to which are attached an amino group (-NH2), an acid group (-COOH), a hydrogen atom (-H), and an -R group. This generic R represents any one of 20 different attachments. There are hundreds of known amino acids, but only 20 that get built into proteins, and -R differentiates each of these 20, depending upon the molecular structure of -R. In the smallest amino acid, glycine, R is a second hydrogen atom, which causes it to have a plane of symmetry between the two H atoms.
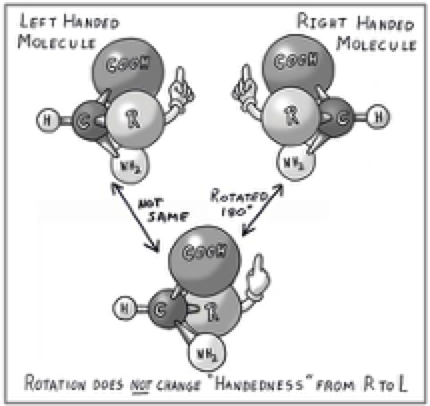 If you can imagine rotating the upper right molecule (labeled “right handed”) 1800 about its vertical axis, it becomes the sketch at the bottom. The gloved hand initially had its palm toward us, but because of this rotation, has gone around to where it has its palm pointing away from us. Nothing has changed. The geometry of the lower drawing is still identical to the upper right version, and both are “nonsuperimposable” mirror images of the one at upper left. (Remember, the amino acid does not really have a gloved hand ─ this is just a figment of artistic license to help keep track.)
If you can imagine rotating the upper right molecule (labeled “right handed”) 1800 about its vertical axis, it becomes the sketch at the bottom. The gloved hand initially had its palm toward us, but because of this rotation, has gone around to where it has its palm pointing away from us. Nothing has changed. The geometry of the lower drawing is still identical to the upper right version, and both are “nonsuperimposable” mirror images of the one at upper left. (Remember, the amino acid does not really have a gloved hand ─ this is just a figment of artistic license to help keep track.)
In the laboratory, when we synthesize an amino acid from symmetrical reagents, the product is always a 50:50 mixture of right-handed and left-handed forms. In living cells, however, only the left-handed amino acids get selected in each and every turn. Does that not seem to imply some geometrical preference? In the laboratory, when we synthesize any sugar, we always get a 50:50 mixture of its two mirror image forms. Yet, when sugars are biosynthesized in plants, no left-handed sugars are formed, only right-handed sugars.
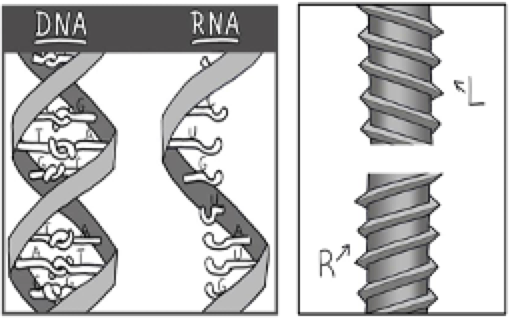 This has to be a consequence of the 3-D geometry of the sugar-forming proteins in the cell having been assembled exclusively from left-handed amino acids. One more piece of this puzzle: DNA and RNA are also asymmetrical. Watson and Crick made their model of DNA using right-handed deoxy-ribose in its helical backbone, and found that this could only form a stable double helix that was right-handed.
This has to be a consequence of the 3-D geometry of the sugar-forming proteins in the cell having been assembled exclusively from left-handed amino acids. One more piece of this puzzle: DNA and RNA are also asymmetrical. Watson and Crick made their model of DNA using right-handed deoxy-ribose in its helical backbone, and found that this could only form a stable double helix that was right-handed.
Compare the last statement with what we see in every hardware store when we need to buy wood screws or nuts and bolts. Since early in the Industrial Revolution, stores agreed never to have left-handed screws, nuts, and bolts in the building, only “righty-tighty” threads. If both kinds were present, imagine the confusion as left-handed types got mixed with right-handed. You rarely encounter any mechanical parts with left-handed threads. When you do, there is a special reason for it. The bolt for the rotor blade of a gas-powered lawn mower is left-handed to ensure that the cutting action tightens the bolt, and does not loosen it. Even stranger, the coupling for attaching a propane gas tank to its hose is always left-handed ─ probably to save you from connecting a garden hose if the original hose is lost!
For our purposes, we have just covered one of the most difficult, yet fascinating, lessons from organic chemistry.
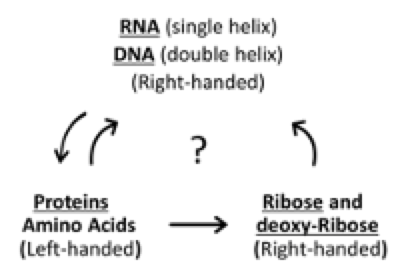 As if the enormous code of DNA, with almost infinite odds against its correct life-defining code ever dialing up by chance, weren’t remotely improbable enough, and
As if the enormous code of DNA, with almost infinite odds against its correct life-defining code ever dialing up by chance, weren’t remotely improbable enough, and- As if the three-way “chicken-egg” conundrum surrounding DNA, RNA, proteins and sugars weren’t too complex to have resulted from random reactions,
- We now see that there is this miraculous three-dimensional requirement that DNA and RNA have right-handed helices ─ so that proteins are biosynthesized exclusively from left-handed amino acids, and now, after all that,
- Only right-handed ribose can properly align RNA in a right-handed helix, to ensure that only left-handed amino acids get selected.
I cannot tell you what you must believe about these relationships. If I tried, you would properly reject the attempt. Only you can make your own decisions about what you fundamentally believe. You may already have a religious faith, or you may be working your way through doubt, based perhaps on science. What I ask you to think about, based on my subject field of organic chemistry, is this. With arguments based on the comparative anatomies that led Darwin to his evolution theory, powerful objections could be raised against any belief in a theistic God, by whatever name the ancients perceived our Creator. Now that we have this molecular evidence about the architectural structure and reactivity of DNA and RNA, and their mutual interdependence with sugars and proteins, we have practically absolute proof of the mechanism for Darwin’s descent with modification. That is good for a belief in evolution, but not so much for atheism. It seems the deeper we go into the incredible complexity of this molecular basis for evolution, the more difficult it becomes to use it as an argument for atheistic belief in a purposeless origin of life.
It is your choice. What you believe is entirely up to you. You are free to conclude that there is no God, and choose instead to believe that complex life is the product of awesome chance. Or you may choose to belief that there is a First Cause for life. We cannot look to science to prove the existence, the power, or the nature of God. Science has no means to approach this question experimentally. But what science can do is reveal to us that life is indeed miraculous in being based upon the elaborate relationships of thousands of chemical substances, so infinitely complex that life is unlikely to have arisen and evolved by unguided chance.
# # #
James G. Martin, Ph.D., was an associate professor of chemistry at his alma mater, Davidson College, from 1960 to 1972. While serving at Davidson, in 1966, he was elected to the Mecklenburg County Board of Commissioners. He served for six years, chairing the body four 4 1/2 years. He was a president of the North Carolina Association of County Commissioners. He then served in the U.S. House of Representatives 1973-1985 and was governor of North Carolina 1985-1993. In 1993 he retired from political life and became chairman of the board of the James Cannon Research Center of Carolinas Medical Center in Charlotte, NC. He is the author of Revelation Through Science, and provided this article and illustraionts as excerpts from that book.
# # #
See related article on the reality of order as evidence of God on this page.
Top image of DNA by Gerd Altmann from Pixabay

